By Emily Leclerc
BU News Service
BOSTON — Cancer is a tough monster to fight. It presents like a hydra with thousands of heads invading every place we don’t want it. A limited number of those heads are currently understood by science, but a newly recognized discovery has provided medicine with a shiny new weapon that may give doctors an edge against cancer.
In 2019, three scientists discovered a molecule that revolutionizes not only the scientific understanding of how cells function, but also how they can develop more precisely targeted cancer therapies.
The Nobel Committee awarded the 2019 Nobel Prize in Physiology or Medicine to three scientists in October for their large body of work on how cells sense and adapt to changing oxygen levels.
Dr. Gregg Semenza, one of the three Nobel laureates, is responsible for the discovery of hypoxia-inducible factor, HIF for short, which is the critical molecule in a cell’s oxygen-sensing pathways. It’s also the hopeful target of future cancer treatments.
“I’m optimistic that within the next several years we will be able to have new therapies [using HIF] that might be useful in treating a number of different cancers,” said Semenza, a member of the John Hopkins Medicine Institute.
Cancerous tumors present a variety of unique issues, one being hypoxia, which is when tissues are starved of oxygen. In tumors, hypoxia relates to the lowered oxygen levels in individual cells.
“Advanced tumors have regions which are very hypoxic because these cells have been pushed so far away from a blood vessel that, [when the blood reaches them], all the oxygen is used up,” Semenza said.
Traditional cancer therapies, such as radiotherapy and chemotherapy, are significantly less effective against cells under the stress of hypoxia, Semenza explained. Because of this, hypoxic cancer cells are able to survive doctors’ current regime of treatments, which can cause the patient to suffer a relapse.
“What we believe is that these hypoxic cells are able to survive cancer therapy because most cancer therapies are targeted to dividing cells,” he said. “The most actively dividing cells are the cells that get plenty of oxygen and are near the blood vessels.”
The newly discovered HIF molecule provides a way to combat hypoxic tumor cells and their ability to survive traditional cancer therapies.
According to Dr. Benjamin Lampson, an instructor of medicine at Dana-Farber Cancer Institute, when a cell experiences low oxygen levels, HIF activates hundreds of thousands of different genes to help the cell adapt to its changing environment.
“[A cell] has to be able to adapt to the levels of oxygen that it sees, and it has to be able to do this quickly,” Lampson said. “It can’t take days for it to adapt. It has to be able to do this within a matter of minutes.”
Dr. Mark W. Dewhirst, a professor of radiation oncology and vice director for basic science at the Duke Cancer Institute, discussed how HIF encourages blood vessel growth to bring more oxygen to the cell.
HIF also controls a cell’s switch from metabolism that requires oxygen to a type of metabolism that does not need oxygen to make energy, Dewhirst explained.
A cell is constantly making HIF, even when it does not need it. Consider HIF to be a river constantly flowing down the side of a mountain, the mountain being the cell.
At normal oxygen levels, a cell breaks down HIF because it does not need it or anything it activates. The cell does this with the use of another molecule called VHL.
VHL, or Von Hippel-Lindau, is responsible for keeping HIF in check when there is plenty of oxygen. VHL is the dam that blocks the flow of the river.
When the bottom of the mountain has plenty of water, the dam holds back the water to prevent flooding. The land does not need more water than it already has. When the bottom of the mountain suffers a drought and is in need of water, the dam senses it and opens up. The river can then flow freely again to bring water to the dry land.
The mechanism that allows VHL to sense HIF, or the dam to sense when water is not needed, is related to oxygen levels.
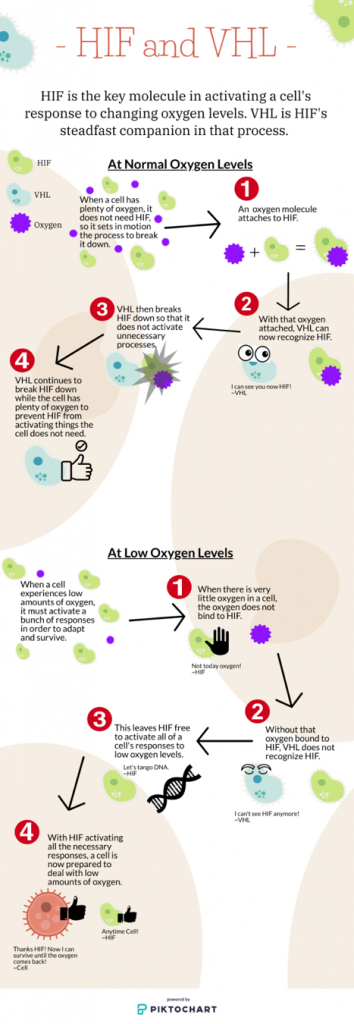
“When there is a lot of oxygen present in the air, part of one of those oxygen molecules is added to the HIF protein,” said Lampson. “VHL can recognize HIF only when an oxygen molecule is attached. When that oxygen molecule is attached, VHL causes [HIF]’s destruction.”
When oxygen levels get low, the oxygen doesn’t bond with HIF, which means VHL can no longer recognize it. “HIF is then not degraded and it remains present in the cell so it can go about turning other genes on to help [the cell] adapt to a low oxygen environment,” Lampson said.
The many and varied responses activated by HIF allow a cell to easily adapt to hypoxia. While important for normal cells, these responses are dangerous in cancerous cells as they can greatly increase the risk of metastasis.
“You have these [hypoxic cancer] cells that have survived,” Semenza said. “They turn on genes that allow them to invade through the tissue, get into a blood vessel, go off to other tissues, and come out of the blood vessel to form another tumor. That is metastasis. For most cancers, it’s the metastatic disease, rather than the primary cancer, that results in mortality.”
HIF is going to be present at high levels in hypoxic cancer cells as they respond to low oxygen levels. This presents an opportunity for uniquely targeted cancer therapy.
Kidney cancer presents a unique case where a HIF inhibiting drug could become an important new treatment. In kidney cancer, the VHL gene is lost, meaning the gene that codes for VHL, the molecule that controls the activation of HIF, is gone.
“It is lost often by mutation or other means, but the VHL protein is no longer present in the cell,” Lampson said. “This is a defining feature of clear cell renal carcinoma (kidney cancer). Over 90% of clear cell renal carcinomas have lost VHL function. It is one of the earliest events in developing kidney cancer cells.”
With VHL no longer present, HIF is not broken down and is essentially given free rein. “HIF being around all the time in a kidney cancer cell causes it to grow. The cancer cell continues to depend on the activity of HIF to grow.” Lampson said. “So that means if you find a way to block the HIF protein, then you have found another treatment for kidney cancer.”
According to the National Institutes of Health, there are several HIF inhibiting drugs currently in clinical trials with the intent of treating kidney cancer. The majority of these trials are looking at how well the HIF inhibitor works alongside chemotherapy drugs.
“We really think that HIF inhibitors, when added to many different existing cancer therapies, may help those therapies work more effectively,” said Semenza. “We’re continuing to do work in animal models. We hope that eventually, we’ll be able to translate that to the clinic.”
The discovery of HIF was monumental and has presented scientists with promising avenues for new and powerful cancer therapies. But there is still much research that needs to be done.
HIF inhibitors are not yet approved for use in America and are still in the throes of clinical trials. There is hope that, in the future, drugs targeting HIF can be utilized in the treatment of many different cancers, as well as in other illnesses.





